Certificate in Translational Science
Navigate Certificate Program in Regulatory, Translational, or Entrepreneurial Science
Translational Science | Regulatory Science | Entrepreneurial Science
Curriculum Includes:
Coursework
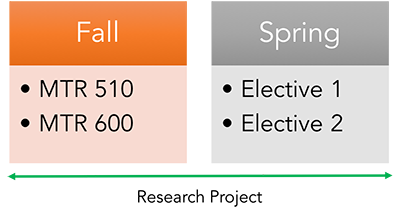 The Certificate Program can be finished in one year and must be completed within two years. The certificate requires the completion of 4 credit units with a passing grade of B- or better. The certificate requires MTR 510 — Introduction to Clinical and Translational Research and MTR 600 — Introduction to Biostatistics. Other biostatistics courses may be substituted if taken in the past two years with a grade of B- or better. The remaining 2 credit units of coursework comprise electives from the certificate. Students may take courses outside of our MTR/REG offerings, but must obtain permission before enrolling.
The Certificate Program can be finished in one year and must be completed within two years. The certificate requires the completion of 4 credit units with a passing grade of B- or better. The certificate requires MTR 510 — Introduction to Clinical and Translational Research and MTR 600 — Introduction to Biostatistics. Other biostatistics courses may be substituted if taken in the past two years with a grade of B- or better. The remaining 2 credit units of coursework comprise electives from the certificate. Students may take courses outside of our MTR/REG offerings, but must obtain permission before enrolling.
Sample Study Plan
Certificate in Translational Science
Develop a strong foundation in the fundamental techniques of translational research.
- MTR 604 Scientific & Ethical Conduct
- MTR 620 Translational Therapeutics
- MTR 642 Building a Life Sciences Starup
- MTR 535 Intro to Bioinfomatics
- REG 618 Introduction to Vaccine Development
- REG 610 Fundamentals of FDA Regulation
- REG 611 Clinical Study Management
- REG 624 Applied Regulatory Processes of Vaccines and Biologics
- REG 612 Intro to Drug Development
- REG 613 Drug Development Decision Criteria
- REG 621 Cell & Gene Therapy
- REG 622 New Trends in Medicine and Vaccine Discovery
- REG 625 Challenges and Controversies in Academic Biomanufacturing
Research Project
The certificate requires a one-year engagement in clinical and translational research. This will take the form of a new research project or as a translational arm to research currently being conducted. Prospective students will identify a mentor and define the research project at the time of application.

