Certificate in Entrepreneurial Science
Navigate Certificate Program in Regulatory, Translational, or Entrepreneurial Science
Entrepreneurial Science | Translational Science | Regulatory Science
Curriculum Includes:
Coursework
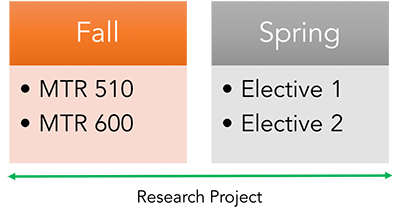 The Certificate Program in Entrepreneurial Science can be finished in one year and must be completed within two years. The certificate requires the completion of 4 credit units with a passing grade of B- or better. The certificate requires MTR 510 — Introduction to Clinical and Translational Research and MTR 600 — Introduction to Biostatistics. Other biostatistics courses may be substituted if taken in the past two years with a grade of B- or better. The remaining 2 credit units of coursework comprise electives from the chosen certificate. Students may take courses outside of our MTR/REG offerings, but must obtain permission before enrolling.
The Certificate Program in Entrepreneurial Science can be finished in one year and must be completed within two years. The certificate requires the completion of 4 credit units with a passing grade of B- or better. The certificate requires MTR 510 — Introduction to Clinical and Translational Research and MTR 600 — Introduction to Biostatistics. Other biostatistics courses may be substituted if taken in the past two years with a grade of B- or better. The remaining 2 credit units of coursework comprise electives from the chosen certificate. Students may take courses outside of our MTR/REG offerings, but must obtain permission before enrolling.
Sample Study Plan
Certificate in Entrepreneurial Science
Translate biomedical research into innovative solutions and develop approaches to commercialization
- MTR 6400 Entrepreneurial Seminar
- HCMG 8670 Healthcare Entrepreneurship
- MTR 6200 Medical Entrepreneurship
- REG 6100 Fundamentals of FDA Regulation
Students may take elective courses outside of these recommendations, by substituting a different MTR/REG course or course outside of our MTR/REG offerings, but must obtain permission before enrolling.
Research Project
The certificate requires a one-year engagement in clinical and translational research. This will take the form of a new research project or as a translational arm to research currently being conducted. Prospective students will identify a mentor and define the research project at the time of application.

